|
home | what's new | other sites | contact | about |
||
|
Word Gems exploring self-realization, sacred personhood, and full humanity
Invisible Morphic Fields Supporting Electron Shells
return to "Evolution" main-page
Electron shells offer strong evidence for subsuming support by invisible morphic fields. Please allow a brief chemistry lesson to help us understand. On the “Bio-Chem-Physics” page I featured Dr. Tyler Dewitt as one of the world’s great teachers. Sample his many youtube videos to learn why he’s so effective. But let’s take note of Tyler’s discussion here.
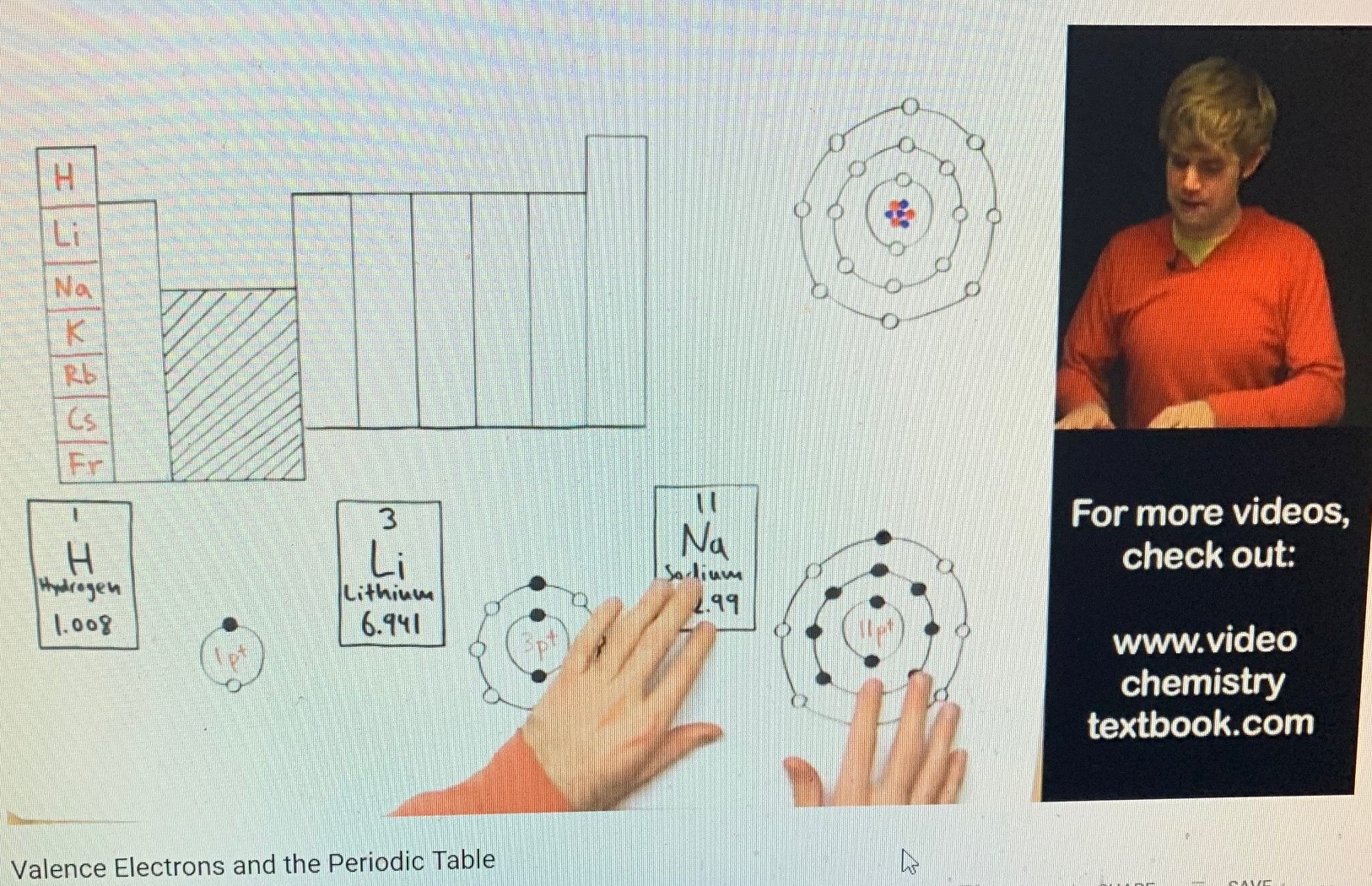
Dr. DeWitt is talking about valence electrons. Valence electrons are the electrons in the outermost shell of an atom. These are the atom’s electrons that facilitate a bonding with other atoms to form compounds. It’s been said that virtually all of chemistry might be defined as the interaction of valence electrons. Featured here are the “Group One” elements of the Periodic Table. See Group One at the far left of the box-diagram of the Periodic Table. In Group One, at the top, we begin with hydrogen; next, beneath hydrogen, we find lithium; and below that is sodium, and so forth, down the line. All of these atoms have something in common. They all have 1 valence electron; that is, they all have only one electron in their respective atom’s outermost shell. See the circular diagrams (above) near Tyler’s hands. Each element – hydrogen (H), lithium (Li), and sodium (NA) – has only one electron (the black dot) in the outmost shell.
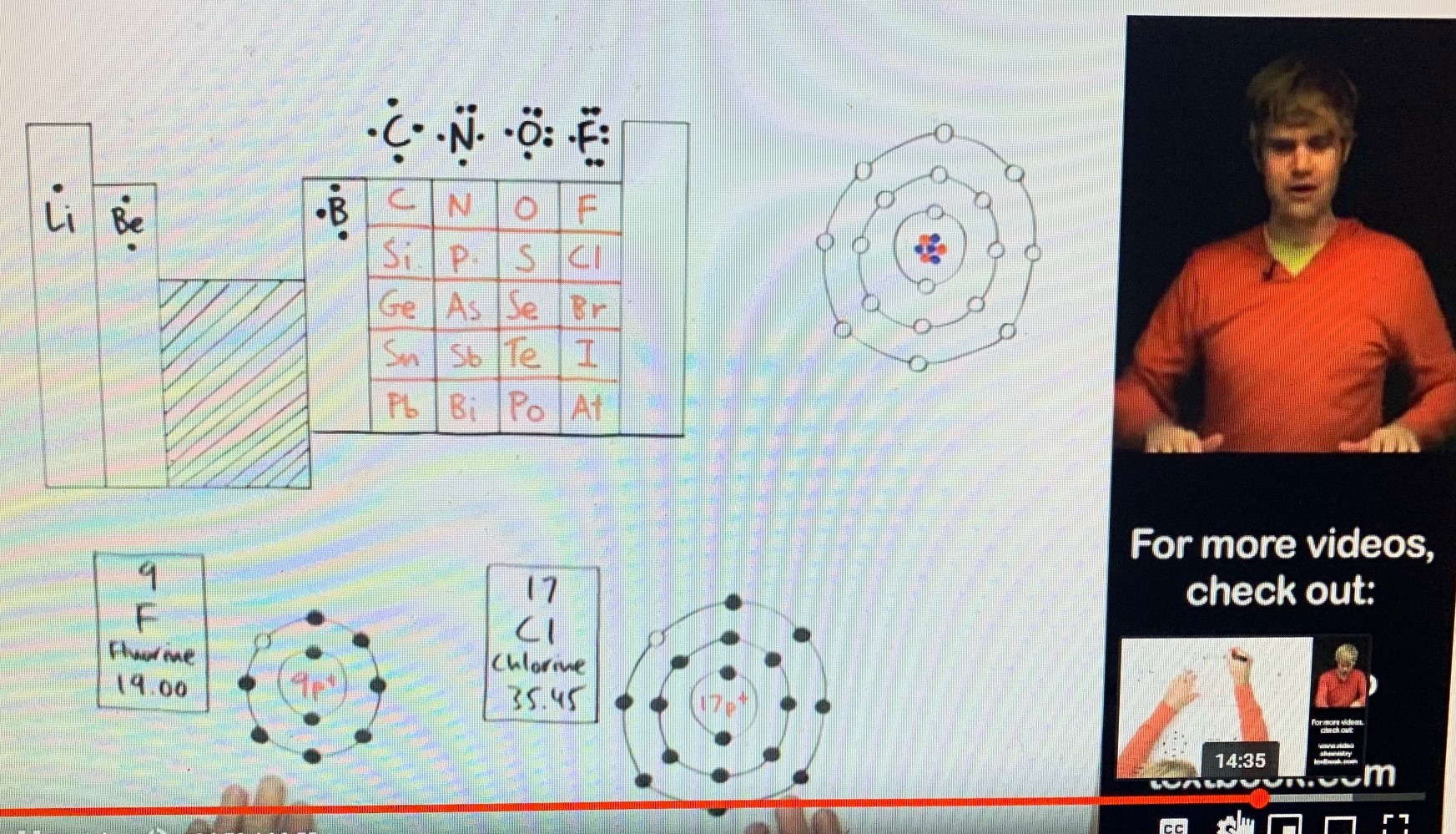
Let’s follow this sequence, from left to right, across the top of the Periodic Table. All elements (atoms) in Group Two have 2 valence electrons. All elements (atoms) in Group Three have 3 valence electrons. And so on, across the top, until we come to Group Seven, which is home to fluorine (F), at the top of the group; then, under fluorine is chlorine (Cl), and so forth, down the list. Again, see the circular diagrams near Dr. DeWitt’s hands, and notice that, each case, there are 7 valence electrons, that is, 7 electrons in the outermost orbital shell. Note, too, that, among the few atoms discussed here – except for hydrogen, which has “2 spaces” in its one and only orbital shell -- there are “8 spaces” for electrons in the outermost shell. This is so common in chemistry (though there are exceptions) that it’s called “The Octet Rule.” Editor’s note: All of this is very interesting, but I must stop here as our purposes do not extend to other implications. However, allow me to add that it is not uncommon for atoms in Group One to easily bond with atoms of Group Seven. Why is this? Atoms “crave” for the stability of full electron shells. To this end, the atoms of Group One would just die to get rid of their single valence electron, which would allow them a remaining “full collector’s set” of a completed orbital(s). And the atoms of Group Seven are just beside themselves (unstable) to acquire a measly extra electron to fill out the remaining space to complete an octet. The classic example of this is the salty love affair of sodium (Na), with 1 valence electron, and chlorine (Cl), with 7 valence electrons. Together, they form a “marriage,” a compound very familiar to all of us, NaCl – common table salt. The amazing thing is, table salt is utterly different, not even close to the properties of the original parent elements. The new compound is “something never seen before.” I’ll be speaking of this hot-chemistry principle in Book Four of the Love In The Afterlife Series, “Omega Point.” But what happens when an atom gives up all of its electrons in an outermost shell? Does the shell continue to exist with “8 empty parking spaces”? In another lecture, Dr. DeWitt responded to this question. His answer was, no, the shell just disappears. He offered an analogy. He said to imagine going to a concert where there’s overflow attendance and people end up parking in a nearby field. He said imagine 8 cars parked on the grass in a row. The row is real. It has 8 cars making up the row. But, at the end of the concert, people come for their cars, drive them away, and now the “row” is simply gone. There are no little markers in the field, on the grass, to say, “park here in this row at the next overflow concert.” All of this is true. If we go hunting for what used to be, for example, sodium’s third orbital shell, once that single electron is “stolen” by chlorine, we’re not going to find it. For all practical purposes, that third shell is gone, without a trace. However, the question remains, could that missing shell return? And if so, what does this say about underlying reality? The fact is, some (or maybe all) compounds, under special circumstances, can be “unraveled.” Original elements can be set free. It’s called “chemical decomposition.” Think of it as a “divorce,” as per our discussion above. When this happens, electrons can be gained or lost. In other words, if an element ends up with less than an octet of valence electrons, where does the new shell come from? It seemingly disappeared when electrons were “stolen” in an earlier bonding with another atom; but if electrons are gained, does a new orbital shell have to be manufactured to accommodate the newly acquired electrons? dancing to the tune of an invisible piper Maybe a clearer example of this fiat creation of shells would be the construction of elements in the "blast furnace" of stars. Under the process of nuclear fusion, all of the elements are built, proton by proton, plus accompanying neutrons and electrons. As the choir grows beyond hydrogen and becomes more complex, how do you "build" another electron shell? - when there was "nothing" there before. Is it so easy that "it just had to be that way"? How do you craft an orderly cosmos out of chaos? - especially, with the antagonist Entropy trying to tear down everything that smacks of harmony, order, and complexity. It seems a bit superfluous, and obvious, to say that these elemental particles are dancing to the tune of some invisible piper. it's the same pattern in trillions of stars Also, regarding these electron shells suddenly appearing in the blast-furnace bellies of stars, we might be persuaded to allow randomness a great voice in the process, but for one major consideration. There are trillions and trillions of stars in the universe, not all of them hot enough to forge the heavier elements, but among those that are hot enough, or have been so, all of the chemical elements created therein, in "cookie cutter" fashion, came off "the assembly line" with identical electron shells, that is, with the same number of "parking spaces" for each orbital level. This could not have happened by chance but speaks to some sort of invisible "blueprint" supervising the "lego construction." they're always there in potentia The clearer view here, as I take it, is that nothing is truly manufactured or lost concerning these shells. I think Dr. Sheldrake is correct to say that there are invisible quantum fields supporting the "behavior" of atomic particles. Like the invisible force-field of a magnet, which iron filings reveal, the electron “shells,” occupied or not, always exist, at least in potentia, supported by hidden morphic fields, which are the unseen "blueprints" of atoms.
|
||
|
|